Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine
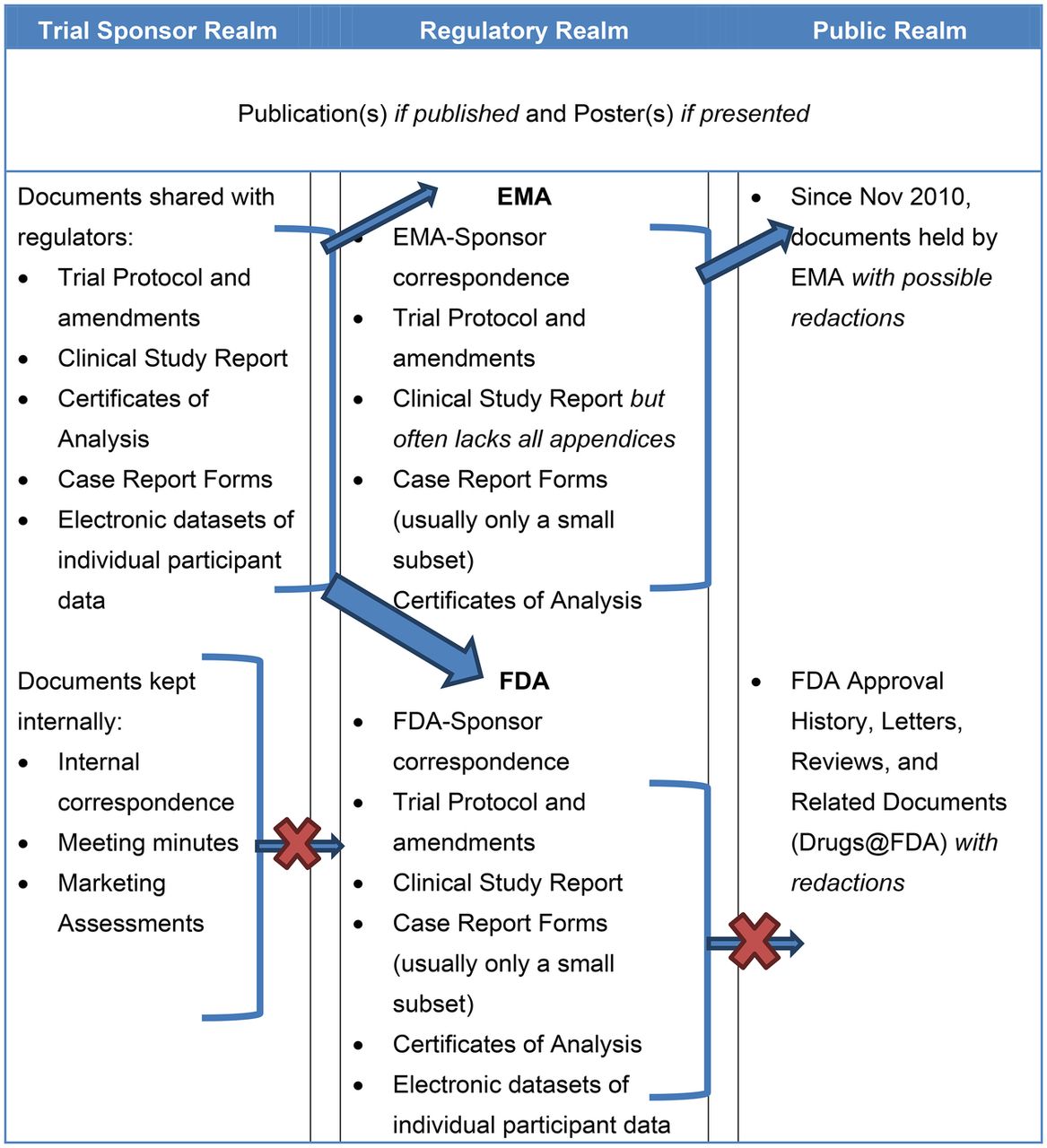
Clinical study reports of randomised controlled trials: an exploratory review of previously confidential industry reports | BMJ Open

Will clinical trial data disclosure reduce incentives to develop new uses of drugs? | Nature Biotechnology

PDF) Abbreviated Clinical Study Reports with Investigational Medicinal Products for Human Use: Current Guidelines and Recommendations

Clinical Trial Report Template (1) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Report template, Professional templates, Clinical trials