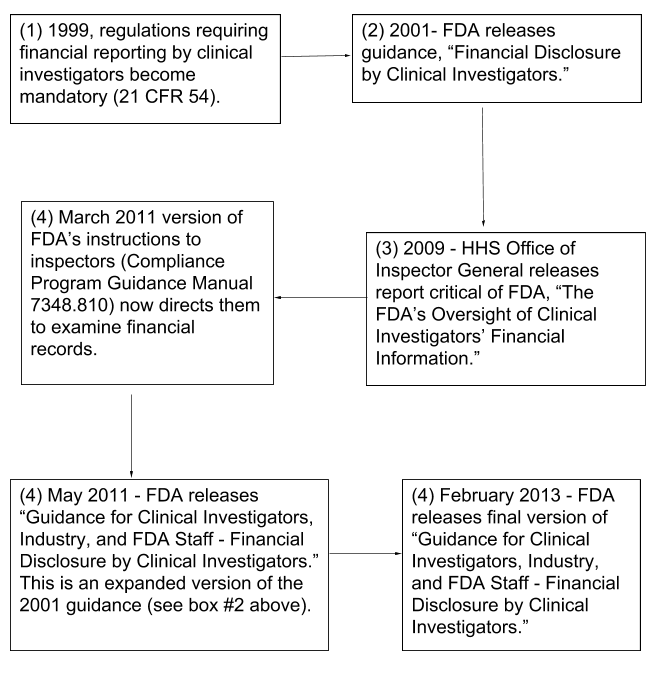
The Food and Drug Administration's Oversight of Clinical Investigators' Financial Information (OEI-05-07-00730; 01/09)

INVESTIGATOR RESPONSIBILITIES April Objectives Review and Discuss: Responsibilities of the clinical research Investigator as per relevant regulations. - ppt download

Form FDA 3455 - Disclosure: Financial Interest and Arrangements of Clinical Investigators Free Download