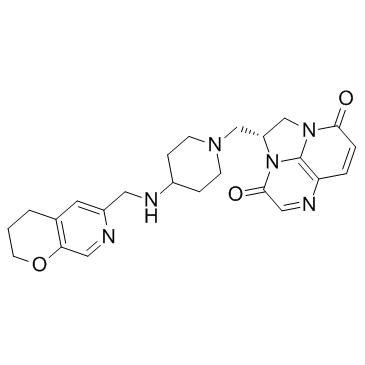
Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK
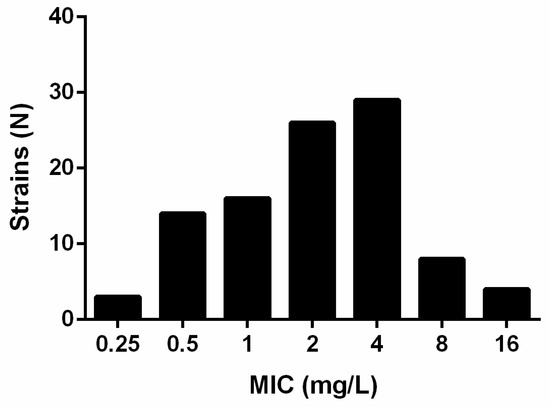
Antibiotics | Free Full-Text | Antibacterial Activity of the Novel Drug Gepotidacin against Stenotrophomonas maltophilia—An In Vitro and In Vivo Study
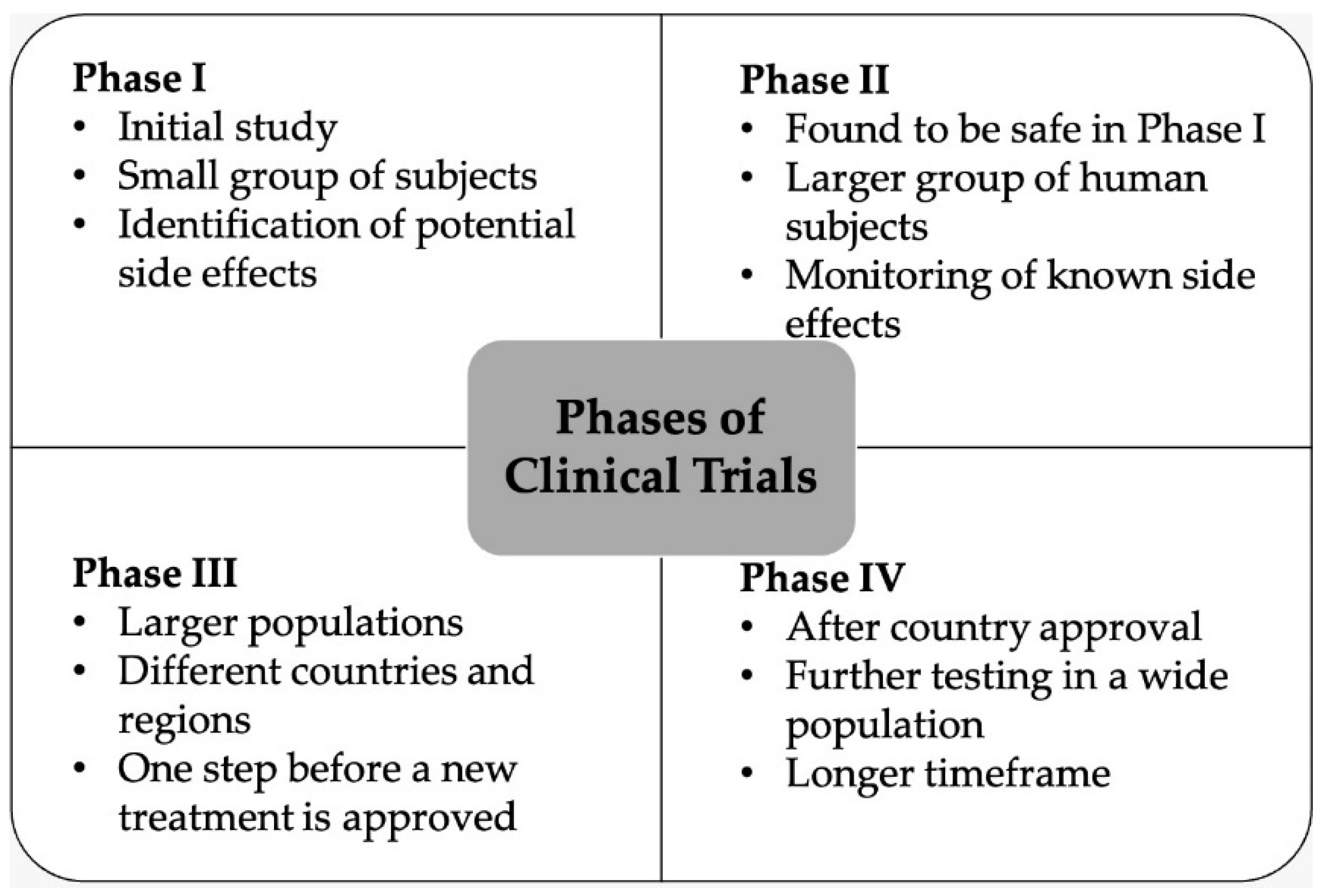
Antibiotics | Free Full-Text | Pharmaceutical Approaches on Antimicrobial Resistance: Prospects and Challenges
First Novel Antibiotic Since 20 Years, Gepotidacin Debuts To Treat Drug Resistance Urinary Tract Infections (UTIs) Amidst Growing Incidences! - Thailand Medical News

Dose Selection for a Phase 3 Study Evaluating Gepotidacin (GSK2140944) in the Treatment of Gonorrhoea | GSK

Mechanistic and Structural Basis for the Actions of the Antibacterial Gepotidacin against Staphylococcus aureus Gyrase | ACS Infectious Diseases

Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK

Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK

Dose Selection for Phase III Clinical Evaluation of Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | Antimicrobial Agents and Chemotherapy
Design of Two Phase III, Randomized, Multicenter Studies Comparing Gepotidacin with Nitrofurantoin for the Treatment of Uncomplicated Urinary Tract Infection in Female Participants | SpringerLink
Phase 2a Pharmacokinetic, Safety, and Exploratory Efficacy Evaluation of Oral Gepotidacin (GSK2140944) in Female Participants wi

Dose selection for a phase III study evaluating gepotidacin (GSK2140944) in the treatment of uncomplicated urogenital gonorrhoea | Sexually Transmitted Infections
Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose- Ranging, Single-Oral Dose Eva

Dose Selection for Phase III Clinical Evaluation of Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | Antimicrobial Agents and Chemotherapy
Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose- Ranging, Single-Oral Dose Eva

Gepotidacin (GSK2140944) Demonstrates Similar Safety and Pharmacokinetics in Adults and Adolescents (12 to ˂18 years) | GSK

Dose Selection for a Phase 3 Study Evaluating Gepotidacin (GSK2140944) in the Treatment of Gonorrhoea | GSK

Overview of microbiome dynamics during gepotidacin Phase 2a clinical... | Download Scientific Diagram